An Introduction to Pain
Introduction to Pain
Pain has been defined by the International Association for the Study of Pain as
“An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage."
Below are some definitions to review.
Pain is the number one reason patients present to physicians. 19% of adults in the United States report persistent pain (defined as constant or frequent pain lasting for at least 3 months).
In a 2014 study by Kennedy et al, rates of persistent pain were highest for
Women
Adults ages 60–69 years
Adults who rated their health as fair to poor
Adults who were overweight or obese
Adults who were hospitalized one or more times in the preceding year.
The Physiology of Pain
Nociceptors
Pains signals are detected by sensory neurons called nociceptors. Nociceptors, also called pain fibers, are neurons that detect damage to tissues and then transmit pain signals to the spinal cord. Different nociceptors detect different types of painful stimuli.
Nociceptors may be myelinated (their axons are insulated by a myelin sheath) or unmyelinated (no myelin sheath around the axon). Recall that myelination increases the conduction velocity, or speed, of signals (i.e., action potentials) as they travel to their destination.
The different types of nociceptors (i.e., pain fibers) are illustrated below.
A-delta Types I and II (myelinated) fibers rapidly transmit signals for "sharp" pain to the spinal cord.
C fibers (unmyelinated) fibers slowly transmit signals for "dull" pain to the spinal cord. C fibers have been implicated in chronic pain.
Nociceptors have free nerve endings (unlike receptors for touch/vibration which have corpuscles) and respond to a range of physical and chemical stimuli.
Normally, nociceptors only respond to signals capable of causing damage. If these receptors become hypersensitive, pain signals can be transmitted even in the absence of tissue damage.
Another type of sensory neuron, called A-Beta fibers, transmit non-noxious mechanical stimuli such as light touch, pressure, and vibration signals to the spinal cord. These fibers are myelinated, so the signal travels fast.
How do Pain Signals Travel to the Brain?
Have you ever stubbed your toe? Immediately after stubbing your toe, your reflexes kick in and you immediately withdraw your foot.
The immediate pain you feel is from the A-delta fibers. Within milliseconds, the dull pain sets in and you REALLY feel it. The delay occurs because C-fibers travel slower than the A-delta fibers.
See the diagram below, which illustrates the fast and slow responses of A-delta and C fibers, respectively.
Tissue damage releases many signaling molecules
After an injury, numerous proteins and signaling molecules are released that immediately initiate the inflammatory process and wound healing process. The substances released from damaged tissues that activate nociceptors are tabulated below.
After a traumatic injury, immune cells and damaged tissue release bradykinins, prostaglandins, potassium, substance P, and histamine which activate nociceptors. Nociceptors have their cell bodies within the dorsal root ganglion and send pain signals to the spinal cord. Because the signals are sent TO the spinal cord/brain, we call them afferent signals (NOTE: signals are called efferent when they are sent from the brain/spinal cord TO the periphery).
After synapsing in the dorsal horn of the spinal cord, the signal is modulated and then sent up the spinal cord to the brain through the anterolateral (i.e., spinothalamic) pathway for further processing.
Pain Modulation in the Spinal Cord: Opioids, Serotonin, and Norepinephrine
The modulation of pain signals occurs in the dorsal horn of the spinal cord. Recall that pain signals are sent from the periphery to the spinal cord via nociceptors. These nociceptors synapse onto other neurons within the dorsal horn of the spinal cord.
Once the pain signals reach the dorsal horn of the spinal cord, the signals are altered and modified by other neurons. These other neurons originate from various areas. Some of them originate from the brainstem and travel down to synapse on, and modify, pain signals.
These descending fibers from the brain stem may be serotonin neurons, norepinephrine neurons, or opioid-producing neurons.
These descending fibers dampen or decrease the intensity of the pain signals before they travel up to the brain. This is why we use serotonin-norepinephrine medications such as duloxetine (Cymbalta) and nortriptyline (Pamelor) for pain management.
There are also nerve fibers, called mechanoreceptors, that originate outside the spinal cord and carry non-painful mechanical signals such as vibration, soft touch, and pressure from muscles and skin. These mechanoreceptors, which are also called A-Beta fibers, also synapse on neurons in the dorsal horn of the spinal cord where they decrease or dampen pain signals. This is why we rub our wounds!
As we've discussed, there are a number of neurons (e.g., mechanoreceptors, serotonin neurons, and norepinephrine neurons) that diminish pain signals in the spinal cord. Opioid-producing neurons are another type of neuron that diminish pain signals.
Endogenous opioids are opioids produced naturally by our bodies. Endogenous opioids play a role in dampening the pain signals in the dorsal horn of the spinal cord. This is why we use opioid medications (e.g., hydrocodone, morphine, fentanyl) for pain management.
See the figures below
The Gate Theory of Pain
As described above, pain signals transmitted by C-fibers (red neuron in the diagrams below) and non-pain signals transmitted by A-Beta fibers (yellow neuron in the diagrams below) arrive at the spinal cord and make a pit stop in the dorsal horn by synapsing on both inhibitory interneurons (red neuron in the diagrams below) and neurons destined for the brain (pink in the diagrams below).
C-fibers and A-beta fibers (i.e., mechanoreceptors) act in opposite ways.
The C-fibers (red neurons in the diagrams below) inhibit the inhibitory interneuron and stimulate the neuron transmitting the pain signal (pink in the diagram below). By inhibiting the inhibitory interneuron, the pain signal is enhanced.
A-beta fibers, which are mechanoreceptors, do the opposite of C-fibers. A-beta fibers (yellow neuron in the diagram below) synapse on inhibitory interneurons (red neuron in the diagram below) and stimulate them. By stimulating the inhibitory interneurons, the pain signal is reduced.
This is why we have a tendency to rub our wounds immediately after we suffer an injury. By rubbing the wound, we dampen the pain signal.
As you can see, the dorsal horn acts like a checkpoint where these pain signals can be modified before traveling up the spinal cord to the brain. We call this the "Gate Theory" of pain. The dorsal horn acts as a "gate" for pain signals.
Perception of Pain
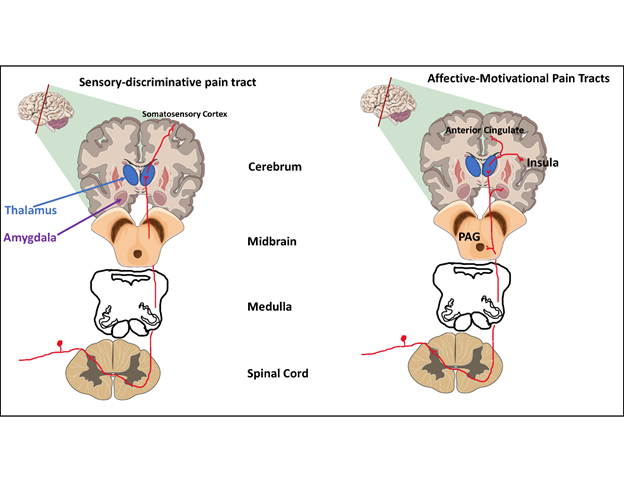
Two separate but parallel pathways send two different types of information.
The Sensory-discriminative pathway sends information about the location of the pain.
The Affective-motivational pathway sends information about the intensity of the pain.
The integration of these two pathways (and others which are not mentioned here) result in the perception of pain. Obviously, this is a very simplified explanation and there is much more complexity, but it gives the learner a basic understanding of how we perceive pain.
Sensory-discriminative
Where does it hurt?
Spinothalamic tract (traditional pain pathway)
Nerve fibers from the Sensory-discriminative tract (spinothalamic tract) are located in the anterolateral region of the spinal cord and synapse in the thalamus. From the thalamus, nerve fibers make their way to the somatosensory cortex where pain is first detected or perceived.
Affective-motivational
How much does it hurt?
Spinoreticular tract
Spinomesencephalic tract
Nerve fibers from the affective motivational tracts are also located in the anterolateral region of the spinal cord and synapse on the reticular formation, periaqueductal gray, amygdala, and medial thalamus.
From there, information is then sent to the "emotional centers" in the cerebral cortex such as the anterior cingulate cortex, insular cortex, prefrontal cortex, and amygdala where pain signals are integrated into an experience.
Brain Regions Involved in Processing Pain Signals: Thalamus, Anterior Cingulate Cortex, Prefrontal Cortex, and Insular Cortex
Pain Tolerance
Pain tolerance occurs on a spectrum. Brain imaging studies have demonstrated that subjects with less pain tolerance have greater activation of the cortical areas discussed previously including the Anterior Cingulate Cortex, Insular cortex, Prefrontal Cortex, and Somatosensory Cortex).
Genetics also plays a role.
Variants of the gene for Catechol-o-methyl transferase (COMT), an enzyme that breaks down catecholamine neurotransmitters such as norepinephrine and dopamine, have been associated with different pain tolerances. Interestingly, individuals with schizophrenia may have a higher tolerance for pain which is supported by the variations in the gene for Catechol-o-methyl transferase (COMT).
References
Arciniegas, Yudisorderfsky, Hales (editors). The American Psychiatric Association Publishing Textbook Of Neuropsychiatry And Clinical Neurosciences. Sixth Edition.
Bear, Mark F.,, Barry W. Connors, and Michael A. Paradiso. Neuroscience: Exploring the Brain. Fourth edition. Philadelphia: Wolters Kluwer, 2016.
Blumenfeld, Hal. Neuroanatomy Through Clinical Cases. 2nd ed. Sunderland, Mass.: Sinauer Associates, 2010.
Cooper, J. R., Bloom, F. E., & Roth, R. H. (2003). The biochemical basis of neuropharmacology (8th ed.). New York, NY, US: Oxford University Press.
Higgins, E. S., & George, M. S. (2019). The neuroscience of clinical psychiatry: the pathophysiology of behavior and mental illness. Philadelphia: Wolters Kluwer.
Iversen, L. L., Iversen, S. D., Bloom, F. E., & Roth, R. H. (2009). Introduction to neuropsychopharmacology. Oxford: Oxford University Press.
Levenson, J. L. (2019). The American Psychiatric Association Publishing textbook of psychosomatic medicine and consultation-liaison psychiatry. Washington, D.C.: American Psychiatric Association Publishing.
Mendez, M. F., Clark, D. L., Boutros, N. N. (2018). The Brain and Behavior: An Introduction to Behavioral Neuroanatomy. United States: Cambridge University Press.
Schatzberg, A. F., & DeBattista, C. (2015). Manual of clinical psychopharmacology. Washington, DC: American Psychiatric Publishing.
Schatzberg, A. F., & Nemeroff, C. B. (2017). The American Psychiatric Association Publishing textbook of psychopharmacology. Arlington, VA: American Psychiatric Association Publishing.
Neuroscience, Sixth Edition. Dale Purves, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, Richard D. Mooney, Michael L. Platt, and Leonard E. White. Oxford University Press. 2018.
Stahl, S. M. (2013). Stahl's essential psychopharmacology: Neuroscientific basis and practical applications (4th ed.). New York, NY, US: Cambridge University Press.
Stern, T. A., Freudenreich, O., Fricchione, G., Rosenbaum, J. F., & Smith, F. A. (2018). Massachusetts General Hospital handbook of general hospital psychiatry. Edinburgh: Elsevier.
Whalen, K., Finkel, R., & Panavelil, T. A. (2015). Lippincotts illustrated reviews: pharmacology. Philadelphia, PA: Wolters Kluwer.
Hales et al. The American Psychiatric Association Publishing Textbook of Psychiatry. 6th Ed.
____
This article was reviewed by a licensed medical professional.

.png)
.png)

.png)


.png)



.png)
.png)
.png)